The term 'generic' word in India denotes the medicines which are marketed under a generic name. Then there is another term called 'Branded generics', to connote medicines which are now off patent and sold under a brand name by companies, this represents almost all the drugs in the Indian Pharmaceutical market. We can supply you branded medicines from India as per your requirement.
Branded Medicines

Generic Medicines
Generic drugs are copies of brand-name drugs that have exactly the same dosage, intended use, effects, side effects, route of administration, risks, safety, and strength as the original drug. In other words, their pharmacological effects are exactly the same as those of their brand-name counterparts. We can supply you generic medicines from India as per your requirement.

Clinical Trial
New tests and treatments aren’t offered to the public as soon as they’re made. They need to be studied. A clinical trial is a type of research that studies a test or treatment given to people. Clinical trials study how safe and helpful tests and treatments are. When found to be safe and helpful, they may become tomorrow’s standard of care.
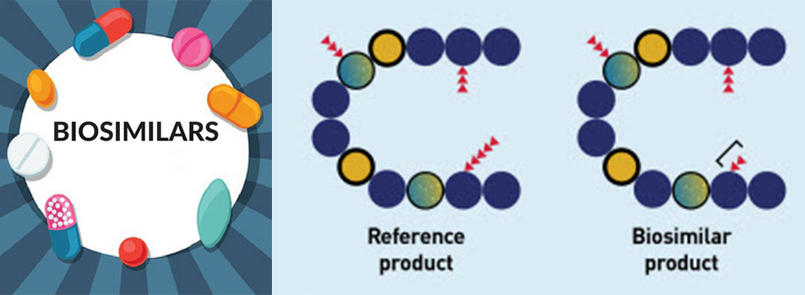
Biosimilar Products
A biosimilar product is a biological product that is approved based on a showing that it is highly similar to an FDA-approved biological product, known as a reference product, and has no clinically meaningful differences in terms of safety and effectiveness from the reference product.

Orphan Drugs
An orphan drug is a pharmaceutical agent developed to treat medical conditions which, because they are so rare, would not be profitable to produce without government assistance. The conditions are referred to as orphan diseases.
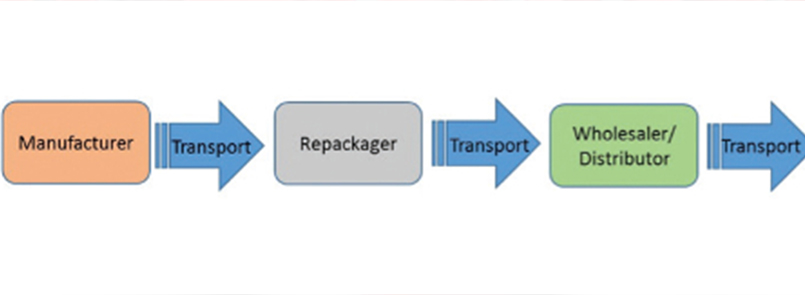
Cold chain Medicines
A cold chain is a temperature-controlled supply chain. It typically involves constant refrigeration of the product from the time of its production through its transportation, handling, storage, and delivery. ... Most biologics require both temperature and time-controlled distribution

Controlled Drugs
Controlled drugs are substances that are controlled under the Controlled Substances Act (CSA). This act categorizes all substances, which are regulated under federal law into “schedules,” depending on how potentially dangerous they are.Jan 20, 2020

Comparator trial
In clinical trials, a comparator drug (defined as 'an investigational or marketed product [i.e. active control] or placebo used as a reference in a clinical trial'[i],[ii]) is often required. Comparator studies typically focus on efficacy relative to a drug that is already on the market, rather than a placebo.
Vaccines
A vaccine is a biological preparation that provides active acquired immunity to a particular infectious disease. A vaccine typically contains an agent that resembles a disease-causing microorganism and is often made from weakened or killed forms of the microbe, its toxins, or one of its surface proteins. We can supply the same.
QUICK BRAND IS COMMITED FOR CLIENT SATISFACTION!
Testimonials
We are very appreciative of Quick Brand and the excellent work that the staff does, I am always amazed at the quick turn-around time when we order something and also am impressed with the support you have given us over the years. Thanks a lot.
Skyler Doe, Happy Customer

We are satisfied with the Product & Services provided by Quick Brand, its with good finishing & technically all parameters are followed by them very accurately. We really recommend them. Best Luck!
Walter White, Happy Customer

We’d like to take a minute to thank for the prompt services. I’ve called on your sales and service techs & always gotten a quick response to my questions. it’s nice to know that we have a supplier that we can count on for all of our manufacturing needs.
Denis Lover, Happy Customer


